Determine Which of the Following Ions Is More Stable.
For example O 2- F - Na Mg 2 and Al 3 all have the electron configuration of Neon. Circle the one more stable structure in each of the following pairs of molecules or ions.
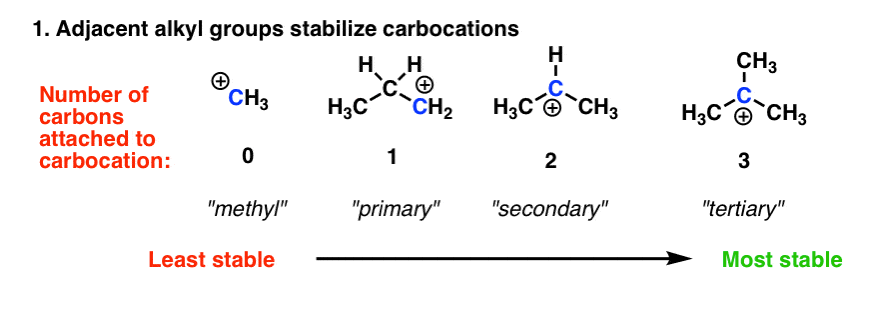
3 Factors That Stabilize Carbocations
Identify the formulas and charges of the cation and anion.
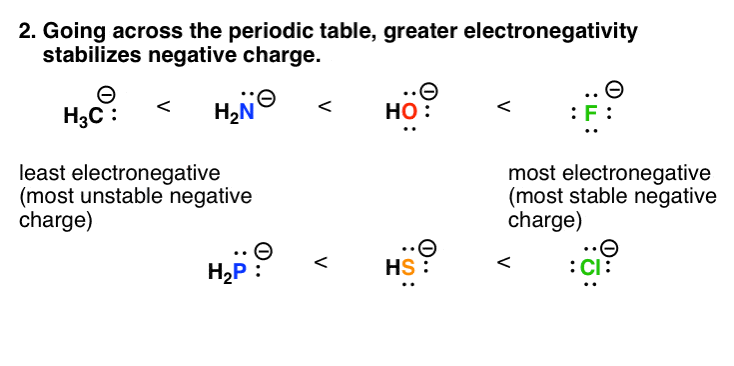
. There are a few principles you need to remember to determine which resonance structure is more stableimportant ie. Determine how many of each ion type is needed to make a neutral compound. An ion is an atom that has a net positive or negative electric charge.
All of the above statements are false. Orbitals is Chromium III ion. Beryllium is in the 2nd group.
The ions having half filled and fully filled electronic configuration is more stable as compared to other ions. Place the cation first in the formula followed by the anion. And for some of these explanations we need to determine the more stable resonance form.
We think so because all the atoms in f have a formal charge of zero. Ionic bonding occurs between a metal which has a high affinity for electrons and a nonmetal which loses electrons relatively easy. Hence among the given ions the only ion which occupies half-filled t 2g.
Eg-Na Electronic configuration 281 After losing one electron It becomes Na ion ansld acquire stability. It has 2 electrons in the outermost energy level. The ions having inert gas configuration are more stable as compared to ion having pseudo inert gas configuration.
Stability of carbocations depends upon the electron releasing inductive effect of groups adjacent to positively charged carbon atom involvement of neighbouring groups in hyperconjugation and resonance. Inspect the following molecules and label each as Z or E isomers if appropriate. So the same number of electrons is Aluminum Al 3 will be bound much closer to the nucleus than the.
Isoelectronic Ions- - ions containing the same number of electrons. Identify two factors that determine the arrangement of ions in an ionic crystal. Is the following sentence true or false.
CaCl2 forms because Ca2 is always a more stable species than the calcium atom alone. 46 5 111 Dhruv00 1 Ions are more stable than parent atom becuase atom loses or gains electron to form ion hence they will acquire octet therefore they are more stable. The exactly half filled and completely filled d - orbitals are extra stableCr3 21 3d3 4s0 -3 unpaired electronsV3 20 3d2 4so -2 unpaired electronsTi3 19 3d1 4s0 -1 unpaired electronMn3 22 3d4 4so - 4 unpaired electronsSo Mn3 ion is most stable in aqueous solution.
Although Mn 3 ion has half-filled t 2g. Stability to transition metal ion is directly proportional to the unpaired electron. Mn 3223d 44s 0t 2g 4e g0.
The most stable ion of it is Be2 ion. However in structure f notice that N has a formal charge of 1 while C has a formal charge of 1- but N is more electronegative than carbon. We know that half-filled and fully filled orbitals are more stable than another half nor fully filled orbitals.
We know that half-filled and fully filled orbitals are more stable than partially filled orbitals. Atoms in general dont like charges so having no charge is better. For a compound to be aromatic it must be cyclic planar have an uninterrupted ring of p orbitals-bearing atoms and have an odd number of pairs of π electrons.
1The concentration of sodium ions are highest in 2The concentration of potassium ions are highest in 3The concentration of phosphate ions are highest in 4The concentration of bicarbonate ions are highest in 5The concentration of protein anions are highest in. Since the negative charge. Each atom ends up with a more stable electron arrangement.
Orbital which reduces the stability. Hence Ca2 is the most stable ion of Ca. If more than one unit of a polyatomic ion is needed place parentheses around the polyatomic ion.
Which of the following ions is more stable. Aromatic compound is more stable than a cyclic compound with localized electrons which is more stable than an antiaromatic compound. Now if we look at Lewis structures e and f with formal charges we can predict with reason that structure e should be stable.
Orbitals is Chromium III ion. Write a reaction mechanism for the addition of hydrogen bromide to 3-methyl-1-butene. But the charge of the nucleus increases from 8 on Oxygen to 13 for Aluminum.
Which of the following ions is more stable. Electronic configuration of Ca21s22s22p63s23p6. CHS it Cr H2 8.
Hence among the given ions the only ion which occupies half-filled t 2g. It contributes more to the resonance hybrid. Mn 3223d 44s 0t 2g.
Which of the following statements are true concerning ionic bonding. Use resonance to explain your answer. 13 Daw at3 or duct for Br CH3 34- 9.
What is the most stable ion of beryllium.

Which Is More Stable Fe2 Or Fe3 Quora
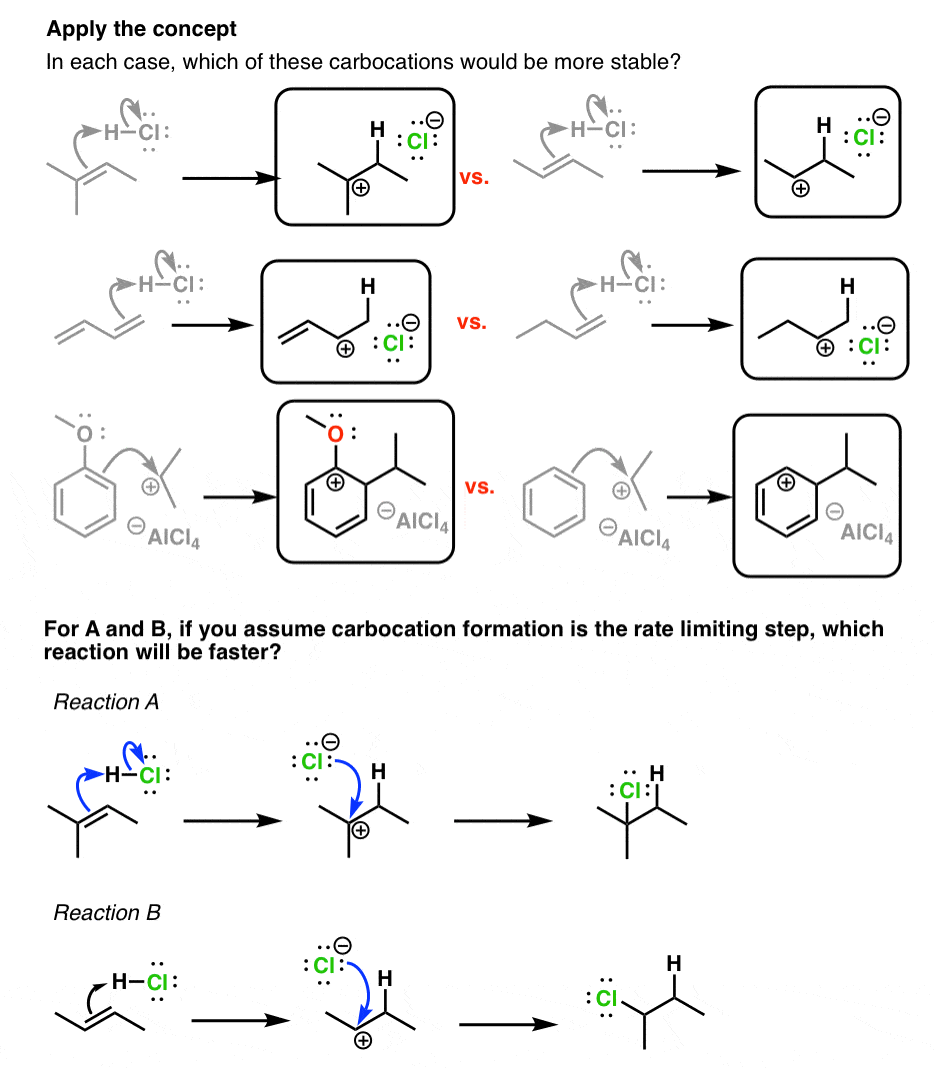
3 Factors That Stabilize Carbocations
Which Is More Stable Fe2 Or Fe3 Quora

7 Factors That Stabilize Negative Charge In Organic Chemistry
0 Response to "Determine Which of the Following Ions Is More Stable."
Post a Comment